For Immediate Release
Company name: DAIICHI SANKYO COMPANY, LIMITED
Representative: Sunao Manabe, Representative Director, President and COO
(Code no.: 4568, First Section, Tokyo Stock Exchange)
Please address inquiries to Noriaki Ishida, Executive Officer,
Vice President, Corporate Communications Department
Telephone: +81-3-6225-1126
http://www.daiichisankyo.com
Daiichi Sankyo’s Subsidiary Daiichi Sankyo Espha to Launch New Generic Drugs
Tokyo, Japan (December 7, 2017) - Daiichi Sankyo Company, Limited (Headquarters Chuo-ku, Tokyo) today announced that its generics subsidiary, Daiichi Sankyo Espha Co., Ltd. (Headquarters Chuo-ku, Tokyo; hereafter, Daiichi Sankyo Espha) will launch in Japan 4 new generic drugs with 2 active ingredients on December 8, 2017, that were announced in the official gazette today.
Overview of Newly Released Products
1. Product Names/Therapeutic Categories
|
Product name
|
Therapeutic category
|
Original brand name
|
|
Rosuvastatin OD Tablets 2.5 mg “DSEP”
|
HMG-CoA reductase inhibitor
|
CrestorⓇ OD Tablets 2.5 mg/
OD Tablets 5 mg
|
|
Rosuvastatin OD Tablets 5 mg “DSEP”
|
|
Famciclovir Tablets 250 mg “DSEP”
|
Anti-herpes virus agent
|
FamvirⓇ Tablets 250 mg
|
|
Famciclovir Tablets 500 mg “DSEP”
|
Rosuvastatin OD(orally disintegrating)Tablets “DSEP”
Rosuvastatin OD Tablets “DSEP” are authorized generics (AG) of CrestorⓇ OD Tablets and will be additional formulations with the same ingredient to Rosuvastatin Tablets 2.5 mg “DSEP” and Rosuvastatin Tablets 5 mg “DSEP” currently sold by Daiichi Sankyo Espha. The launch of the OD tablets will be same line with the original brand product regarding available formulations and specifications.
2. Product Attributes
Daiichi Sankyo Espha has achieved formulation and labeling innovations. They will reduce the burden on the pharmacist in checks to prevent medication dispensing errors and avoid medical accidents such as patients taking the wrong medicine by mistake.
① Innovative design of tablets (Famciclovir tablets)
a. Double-sided printing
Information including product name and active ingredient content is printed on both sides by ink jet printing to make the tablets easy to identify.
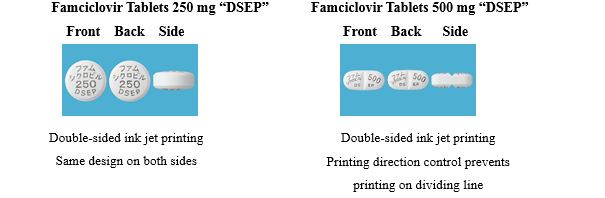
b. Tablet size and specifications:
To improve medication adherence, Famciclovir Tablets 250 mg have been reduced in size to make them easier to take and Famciclovir Tablets 500 mg have been given specifications based on the dosage and administration directions for Herpes zoster.
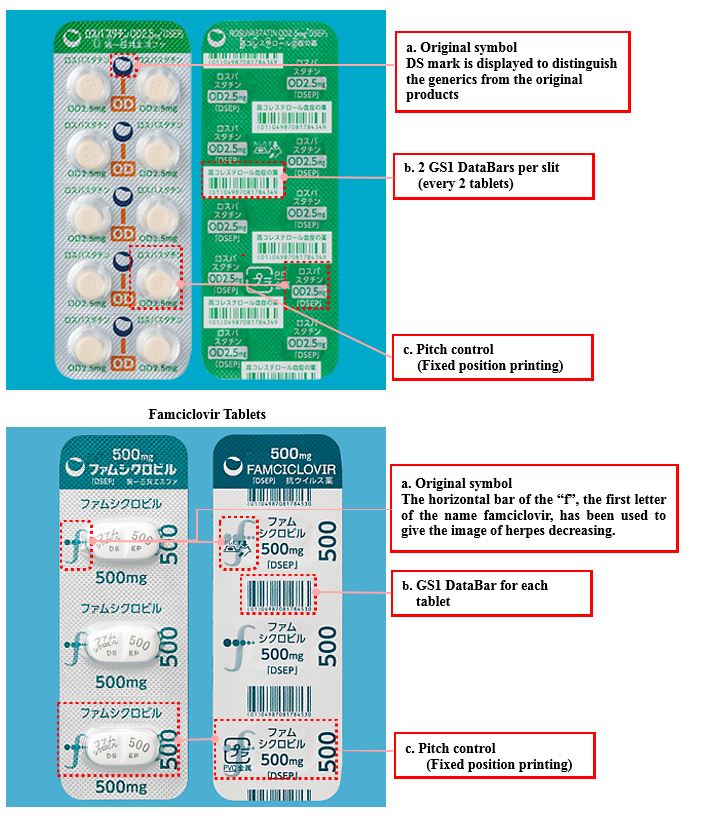
② Innovative design of PTP sheets (Rosuvastatin OD Tablets, Famciclovir Tablets)
a. Original, easily recognizable symbols
The original symbol is displayed on each section of the surface of the PTP sheet to make the tablets easy to identify.
b. GS1 DataBar
A GS1 DataBar is displayed on the back of the PTP sheet to reduce the burden on pharmacists seeking to avoid mistakes when dispensing.
|
GS1 DataBar
A next generation bar code adopted as a global standard in 2010. The GS1 DataBar (sales/original carton unit) not only enables management of the product name, content, volume and number of tablets per sheet but also that of the production lot and expiration date.
|
c. Pitch control (Fixed position printing)
Facilitates identification of the product name, active ingredient and the name “DSEP”
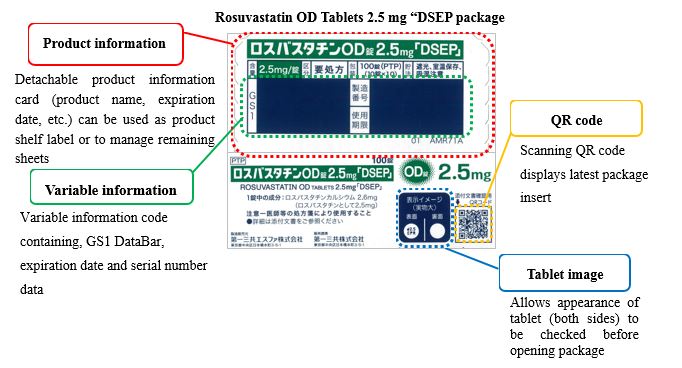
③ Innovative box design
Some of the new products, will be in “iPackage” boxes that provide various information for confirmation at the time of dispensing.
About Daiichi Sankyo Espha
Based on the spirit of the Daiichi Sankyo Group corporate mission* of supplying pharmaceuticals to address diverse medical needs, Daiichi Sankyo Espha provides innovative high value-added generic drugs characterized by ingenious formulations and labeling as well as authorized generic drugs, which are manufactured from the same substances and additives, with the same manufacturing methods, in the same plant as the original drugs. Daiichi Sankyo Espha strives to provide pharmaceuticals that offer users peace of mind by fulfilling the most important pharmaceutical criteria of quality, information, and stable supply while delivering the economic benefits of generic drugs.
*Daiichi Sankyo Group corporate mission: To contribute to the enrichment of quality of life around the world through the creation of innovative pharmaceuticals and through the provision of pharmaceuticals addressing diverse medical needs.
Daiichi Sankyo Espha Company Overview
Company name: Daiichi Sankyo Espha Co., Ltd.
Established: April 1, 2010
Business: Manufacture and sale of pharmaceuticals
Capital: 450 million yen
Representative: President Hiroto Yoshiwaka
Headquarters: 3-5-1, Nihonbashi, Honcho, Chuo-ku, Tokyo
Website: http://www.daiichisankyo-ep.co.jp/ (Japanese)