For Immediate Release
Company name: DAIICHI SANKYO COMPANY, LIMITED
Representative: Takashi Shoda, President and Representative Director
(Code no.: 4568, First Section, Tokyo, Osaka and Nagoya Stock Exchanges)
Please address inquiries to Toshiaki Sai, General Manager,
Corporate Communications Department
Telephone: +81-3-6225-1126
http://www.daiichisankyo.com/
Daiichi Sankyo Announces Organizational Restructuring as of April 1, 2010
Tokyo, Japan (February 26, 2010) - Daiichi Sankyo Company, Limited (hereafter; Daiichi Sankyo), today announced the following organizational changes beginning on April 1, 2010.
DAIICHI SANKYO CO., LTD.
Daiichi Sankyo has been implementing its first mid-term business management plan for FY2007 to FY2009 under its vision to become a 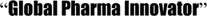 by 2015. The 3-year plan calls for creation of a global management framework, particularly for value chain functions, to ensure optimization throughout the organization.
by 2015. The 3-year plan calls for creation of a global management framework, particularly for value chain functions, to ensure optimization throughout the organization.
Considering the intensifying competition and rapidly changing business environment, now more than ever it has become important to further enhance our corporate value by executing effective and efficient business operations through the optimal combination of regional priorities and global functionalities.
Following are the major components of the new structure.
1. Corporate functions within a global management structure
a) Corporate strategy function
The Licensing Department, Global Marketing Department and Intellectual Property Department will handle all types of planning, promotion and implementation of Daiichi Sankyo Group strategy.
Additionally, staff will be assigned to encompass strategies for management, human resources, IT, and corporate social responsibility.
b) Corporate business management function
The Corporate Finance & Accounting Department, Corporate Communications Department, Internal Audit Department, and the Secretariat Department will handle all types of planning, promotion and implementation for the Group's financial strategy, and will manage the overall business operation of the Group.
Additionally, a Corporate Business Management Staff team will be formed and the Corporate Strategy Department and the International Business Management Department will be dissolved.
2. Japan Company
This virtual company's mission is to consolidate and increase the value of the Japan business. Japan Company will comprise the existing Sales & Marketing Division and the newly created Administration Division and Business Intelligence Division.
a) Establishment of Administration Division
This unit will participate in decision-making for domestic operations, integrating the management of Daiichi Sankyo Co., Ltd. and domestic Group companies and providing business support. It will consist of the Corporate Business Management Department, the Human Resources Department, the IT Strategy Department, the General Affairs Department, and the Legal Affairs Department.
b) Establishment of Business Intelligence Division
This division will function outside marketing operations and manage such domestic businesses as post-marketing quality assurance (QA) in Japan. It will retain the External Affairs Department, the Product Lifecycle Management Department, the Post-Marketing Study Department, the Product Information Management Department, and the Vaccine Business Planning Department.
3. ASCA* Company
This unit will oversee businesses in Asia (excluding Japan) and South and Central America. The company's mission will be to pursue a mid and long-term strategy of growth and increase the value of the Group's business in these regions. Establishment of ASCA Company will dissolve the International Business Management Department.
*ASCA, which stands for Asia South & Central America, is an internal name.
a) Establishment of Corporate Business Management Department
This unit will oversee medium-term and annual business planning, company-specific business planning and corporate administration of ASCA region.
b) Establishment of Marketing Department
This department will foster brand marketing, oversee regional strategies and ASCA licensees and agents, and support Group company operations.
4. Global Functional Divisions
a) R&D Division
i. Establishment of Lead Discovery Research Laboratories I and Lead Discovery Research Laboratories II
The Exploratory Research Laboratories I and Medicinal Chemistry Research Laboratories I and II will be overhauled to establish the new Lead Discovery Research Laboratories.
Lead Discovery Research Laboratories I will handle the compilation and management of
Daiichi Sankyo's natural substance library and Lead Discovery Research Laboratories II will
consolidate structure-based drug design and fragment-based drug discovery*.
*Structure-Based Drug Design/Fragment-Based Drug Discovery: Virtual screening drug design methods that rely on computer modeling techniques
ii. Establishment of Oncology Research Laboratories and Cardiovascular-Metabolics Research Laboratories
Reviewing current disease-centered functions, Daiichi Sankyo will concentrate on the oncology and cardiovascular fields. Biological Research Laboratories I, II and IV and Exploratory Research Laboratories II will be restructured to create the Oncology Research Laboratories and Cardiovascular-Metabolics Research Laboratories.
iii. Establishment of Frontier Research Laboratories and Biological Research Laboratories
Daiichi Sankyo will overhaul the Biologics Research Laboratories, Exploratory Research Laboratories II, and Biological Research Laboratories III to establish the Frontier Research Laboratories. This new entity will study new diseases in areas other than cancer and in cardiovascular fields with a first-in-class focus. The Biological Research Laboratories will also be set up to explore late clinical stage diseases and provide development support and life cycle management for drugs already in the market.
With the overhaul, Exploratory Research Laboratories I and II, Medicinal Chemistry ResearchLaboratories I and II and Biological Research Laboratories I through IV will be dissolved.
b) Pharmaceutical Technology Division
i. Establishment of Biopharmaceutical Technology Research Laboratories
Complementing policies to reinforce its antibody drug businesses, Daiichi Sankyo will combine bio manufacturing technology research from the Process Technology Research Laboratories with that of Asubio Pharma to create the Biopharmaceutical Technology Research Laboratories.
This will enable the company to handle manufacturing research for new biodrugs at Asubio.
c) Supply Chain Division
i. Reorganization of the Supply Chain Planning Department
The Supply Chain Management Department will be merged with the Supply Chain Planning Department, terminating the Supply Chain Management Department.
ii. Establishment of Supply Chain Technology Department
The Supply Chain Technology Department will be created to ensure that the numerous transfers of goods relating to reorganizations of domestic formulating plants proceed smoothly and efficiently.
d) Quality and Safety Management Division
This unit will comprise of the Post-Marketing Regulatory Affairs Department, Quality Assurance Department, Pharmacovigilance Department, and R&D Quality Assurance Department following the transfer of the Post-Marketing Study Department and Product Information Management Department to the Business Intelligence Division in Japan Company.
DAIICHI SANKYO PROPHARMA CO., LTD.
1. Handover of Shizuoka plant
Daiichi Sankyo Propharma will hand over its Shizuoka plant to CMIC Co., Ltd.
2. Establishment of Tatebayashi plant
The Tatebayashi plant will be set up to facilitate the transfer of biopharmaceutical manufacturing from the Tatebayashi BioPharma Center in line with the restructuring of Asubio Pharma.
DAIICHI SAKYO CHEMICAL PHARMA CO.,LTD.
The General Affairs Department will be dissolved after being consolidated with the General Administration Department.
ASUBIO PHARMA CO., LTD.
The following groups will be assigned to manage training of personnel, to improve expertise and to promote new drug discovery themes as the Daiichi Sankyo Group restructures its drug discovery venture business:
Faculty of Corporate Administration, Faculty of R&D Administration, Faculty of R&D Planning, Faculty of Chemistry & CMC, Faculty of Pharmacology I, Faculty of Pharmacology II, Faculty of Safety &ADME, Faculty of Discovery & Biotechnology I, Faculty of Discovery & II, Faculty of Clinical Research & Development
End