Aiming to Be a Trusted Medical Partner
In Japan, Medical Representatives (MRs) play a role in providing, gathering, and disseminating information on our pharmaceuticals to healthcare professionals including doctors and pharmacists.
We handle a wide range of drugs for the treatment of diseases including cardiovascular diseases such as thrombosis and embolism; lifestyle-related diseases such as hypertension and diabetes mellitus ; central nervous system diseases such as migraine and epilepsy; pain; and cancer. Through a well-developed training system, we try to acquire not only information on our own pharmaceuticals, but also a range of knowledge about diseases and pathological conditions. From professional information on the safety and efficacy of drugs that is useful for healthcare professionals who treat patients with various diseases, to information that helps appropriate patients who take our medicines lead healthy and fulfilling lives with their families, we commit ourselves to communicating information that meets the wide-ranging needs of healthcare professionals in an accurate, prompt and thorough manner.
In addition, we are committed to dissemination of information about our products in a compliance manner. This is done through a compliance system that includes training, review of materials and information, etc.
As a result of this ongoing effort, in a survey conducted on healthcare professionals by third-party research firms aimed at continual improvements in such MR activities, Daiichi Sankyo was ranked No. 1 in an overall assessment of MR activities in Japan in both, the entire market and the hospital and private-practice market categories in fiscal year 2023 as well.
We aim to be a trusted medical partner through providing tailored information to all healthcare professionals and their patients, with compassion for each and every one of them.
Assessment by Questionnaire in Japan
| Entire markets (all responding physicians) |
No. 1
(N=4,226) |
| Hospital market (hospital physicians) |
No. 1
(N=2,583) |
| Private practice market (private practice physicians) |
No. 1
(N=1,643 |
Conducted by INTAGE Healthcare Inc. (Surveyed in February 2024)
※ The surveys have been conducted by the same company since 2016 although the company has changed its name from ANTERIO Inc.
Provision of High-quality Information to Healthcare Professionals
Given that pharmaceuticals by nature inevitably have both benefits and risks that must be carefully balanced, it is important for pharmaceutical companies to generate high-quality information on the pharmaceuticals’ efficacy and safety, and to provide the information to the medical community in order to promote their proper use. In particular, in the early phase after the launch of a new drug, there may be less information available to meet various needs in the medical practice, even though the efficacy and safety of the drug was confirmed in the development phase. Through cooperation among relevant units, the Daiichi Sankyo Group generates information through identifying necessary and helpful information from professional viewpoints and conducting post-marketing surveillance, testing, etc.*1 with cooperation from healthcare professionals. We aim to contribute to medical treatment and promote proper use by sharing this information on a timely basis with healthcare professionals through medical journals, conference presentations, and proper use materials.
We continually ensure compliance with relevant laws, regulations and guidelines, as well as ethical and scientific principles, when conducting post-marketing surveillance, testing, etc. We also strive to ensure transparency and manage conflicts of interest by working with impartial third-party medical institutions.
*1Surveys or tests conducted by holders of marketing authorization to collect, detect, confirm, or verify information related to the quality, efficacy, and safety of pharmaceuticals, as defined in the ministerial ordinance on GPSP (Good Post-Marketing Study Practice).
Collection of Information from Healthcare Professionals and its Feedback
We collect more than 37,000 case reports annually from healthcare professionals in Japan on the safety of marketed and investigational drugs, including adverse drug reactions, and approximately 135,000 case reports annually when information from overseas partners is included (number based on FY2024 results). The Safety and Risk Management Department enters the information into the company’s global safety database management system to evaluate it and promptly send reports on findings to the regulatory authorities based on regulatory requirements. Furthermore, once the safety information is analyzed globally, the latest findings are provided as feedback to healthcare professionals.
An enlargeable image opens in a separate window
Response to Inquiries from Patients and Healthcare Professionals
The Medical Information Center in Japan handles inquiries about our pharmaceutical products from healthcare professionals and patients each day, approximately 5,000 inquiries per month, or 60,000 per year. For each of these inquiries, we are committed to complying with all relevant laws and industry codes, and strive to respond sincerely and with consideration for each person making the inquiry. From FY2022, we reorganized the Medical Information Center to a domain-based system of responders to provide more specialized support. Experts in specific fields now handle inquiries directly, allowing us to respond more precisely and promptly than ever. In addition, we undergo daily training in questioning and explanation skills as well as in medical and pharmaceutical knowledge so that we can investigate drug information based on an understanding of the background of the inquiry and provide accurate and easy-to-understand explanations.
We have introduced many technologies and systems, including artificial intelligence (AI), and are utilizing them to respond to inquiries. These include systems that instantly present the most appropriate Q&A to the responders, and a voice recognition system that directly connects the caller to a responder with expertise in each product inquired about. We have also introduced a system that allows us to respond to inquiries even in a remote work environment, so that we can continue to respond as much as possible under any circumstances.
In October 2021, we newly launched an AI-based DI chatbot, “Anytime DI24,” on our website for healthcare professionals so that they can obtain information without having to call the Medical Information Center. Alongside our existing FAQ section (in Japanese only), this allows access to accurate information 24/7/365.
As a result of these initiatives, we have received the No. 1 overall satisfaction rating in a call center evaluation survey*2 of health insurance pharmacists in Japan for nine consecutive years from FY2015 through FY2023.
The Medical Information Center will continue to contribute to healthcare not only by responding to inquiries from healthcare professionals and patients, but also by sharing the inquiries and requests as valuable feedback within the Company for further improvement, and by creating an environment where product information can be obtained and used accurately, quickly, and conveniently through the integration of human and digital technologies.
*2Outsourced to an external research firm
【VOICE】 Aiming to Provide an Inquiry Response that Meets the Expectations of the Person Making the Inquiry

Hiroko Nakamura
Cancer and Narcotics Group
Medical Information Department
Sales Division, Japan Business Unit
Daiichi Sankyo Co., Ltd.
The Medical Information Center is responsible for responding to inquiries about our products from healthcare professionals and patients. We are committed to providing highly specialized information, improving the quality of our response, and utilizing customer feedback with the aim of responding to inquiries in a way that is attentive to those who contact us and meet their expectations. We respond to inquiries on a daily basis with the goal of providing information that is useful to our customers. We strive to understand why they approach us and what their true needs are. We also strive to acquire knowledge through various training programs so that we can provide information with a higher level of expertise.
Ninety-nine percent of all inquiries from healthcare professionals and patients are received by phone. We are working to improve the quality of our response so that we can listen to our customers closely and speak sincerely with not only those who make inquiries, but also those who are affected by our response. In some cases, the valuable feedback we received led to improvements in product development and formulations. We will remain committed to responding to inquiries in a way that meets expectations from customers so that we can contribute to solving the problems of those who make inquiries and become a trusted healthcare partner.
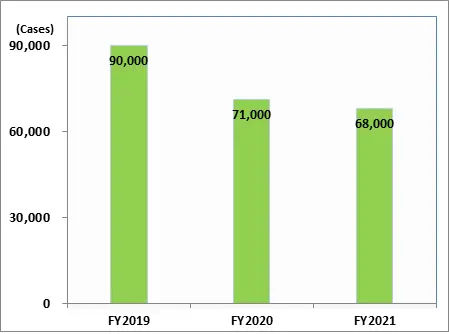
Number of Inquiries Received (Patients, Healthcare Professionals)
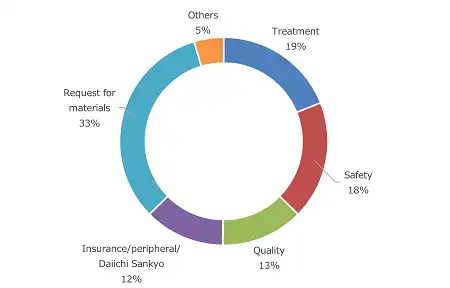
Breakdown of Inquiries by Content (Fiscal 2023)
System to Utilize Customer Feedback
We see Voice of Customers (VOC), received from patients and healthcare professionals, as the most valuable information of all. Every day, we carefully collect, analyze, and assess this feedback at our Medical Information Center. This information is quickly shared within the Company through the VOC portal, helping identify new issues and propose improvements.
We uncover the varied healthcare needs extracted from this information and the clinical questions behind the inquiries, then find clues to resolve them. Finally, we reflect the results to improvements in product formulation and packaging to create concrete solutions. Some of these stories are shared on the “Turning Our Customer’s Voices into Reality” section of the Daiichi Sankyo website.
We believe our mission is to quickly adapt to changes in the healthcare environment and contribute to society by creating better products that reflect customer feedback. We will continue to value the feedback of all our stakeholders as we strive to make better products.
Please visit here for the article “Minasama-no-Koe wo Katachi ni” (Turning Our Customer’s Voices into Reality).
Proposal of product concepts standing close to patients’ and healthcare professionals’ opinion
Through communication with patients and healthcare professionals, Daiichi Sankyo seeks to develop formulations that adds values including usability, reassurance, and satisfaction with consideration for the true needs in healthcare settings. For example, an anti-influenza virus agent, Inavir nebulizer formulation, was launched in October 2019, in addition to Inavir Dry Powder Inhaler. Inavir nebulizer was developed to enable patients with difficulties in taking existing dry powder inhalers, such as children and the elderly, to inhale the misted medicine by natural breathing. On top of that, the disposable inhaler is applied to save time and effort of disinfection for healthcare professionals, and to reduce risk of infection for patients and healthcare professionals.
In this way, we will continue to hone our pharmaceutical technology and contribute to patients and healthcare professionals by improving usability, reassurance, and satisfaction.
Formulation, Labeling and Packaging Schemes for Easier-to-Swallow Medication and Prevention of Medication Errors
Daiichi Sankyo Espha offers generics that are easy to swallow and prevent medication errors.
For example, we improved the distinguishability of tablets by printing the drug name on both sides and labeling the PTP sheets with barcodes and original symbols. In addition, Daiichi Sankyo Espha developed innovative labeling called “iPackage” on the individual packaging box. The iPackage features the following: (1) Detachable information card with code involving expiration date, manufacturing number that can be used as a product shelf label or to manage remaining sheets; (2) Tablet image that allows appearance of tablet to be checked before opening the package; and (3) a QR code that displays the package insert when scanned.
Several AGs*4 that we launched also incorporate double-sided printing on tablets and the iPackage design.
There are cases where the family members of patients, especially small children, take relatively high risk medicines such as anticancer drugs by mistake. Daiichi Sankyo Espha developed outer packaging for PTP sheets (C-Guard/Child Guard) for the purpose of preventing people from accidentally touching drugs and drugs from falling out, with the added feature that it prevents accidental ingestion by small children.
*4Authorized generics: Generic drug manufactured after receiving license from the brand-name pharmaceutical company